Physical Properties of Alcohols
Physical Properties of Alcohols: Overview
This topic covers concepts such as Physical Properties of Alcohols, Water Solubility of Alcohols, Boiling Point of Alcohols, and Azeotropic Distillation of Alcohols.
Important Questions on Physical Properties of Alcohols
Given below are two statements : One is labelled as and the other is labelled as .
Butan––ol has higher boiling point than ethoxyethane.
Extensive hydrogen bonding leads to stronger association of molecules.
In the light of the above statements, choose the correct answer from the options given below :
In allylic and benzylic alchohols, group is attached to
Assertion: Butanol has highest boiling point than ethoxyethane.
Reason: Butanol exhibits intermolecular hydrogen bonding.
Lower alcohols are miscible with water because of the fact that _____
Assertion (A): An ether is more volatile than an alcohol of comparable molecular mass.
Reason (R): Ethers are polar in nature
Which is most VISCOUS ?
Arrange the following compounds in order of their increasing boiling points:
Alcohol which is most soluble in water is
Which of the following has highest boiling point?
The % composition of ethanol in azeotropic mixture is _____.
Ethanol and water mixture is known as _____ azeotropes.
Mention the composition of ethanol azeotropes.
How does azeotropic mixture purified?
What are entrainer distillation of azeotropic mixture?
Arrange the following compounds in increasing order of boiling point:
Propan--ol, butan--ol, butan--ol, pentan--ol
Arrange the following compounds in order of their increasing boiling points n-butyl alcohol, glycerol, n-butane, tert-butyl alcohol, sorbitol, n-butyraldehyde, isobutyl alcohol.
Explain the following :
Methanol is more soluble in water than Propan--ol.Explain the following :
Propan--ol has higher boiling point than n-Butane.
Alcohols are soluble in water whereas ethyl ether is not. Explain.
How many of the following compounds will have higher boiling point than butanol?
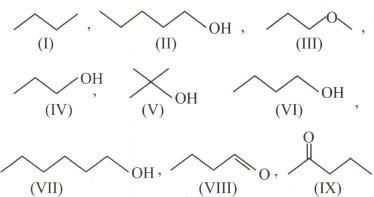
.
